09/5/1: Difference between revisions
No edit summary |
No edit summary |
||
| Line 26: | Line 26: | ||
Ionic conductivity of electrolyte layer was calculated from data obtained from electrochemical impedance spectroscopy (EIS) measurements. R value was determined from Nyquist`s plot. | Ionic conductivity of electrolyte layer was calculated from data obtained from electrochemical impedance spectroscopy (EIS) measurements. R value was determined from Nyquist`s plot. | ||
<math>κ=\frac{d(cm)}{S(cm^2)×R(Ω)}\,</math> | |||
Revision as of 13:16, 3 May 2009
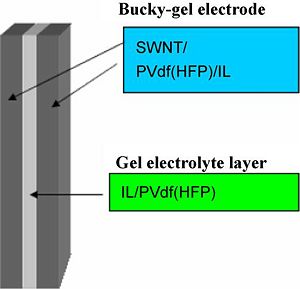
Preparation of 3-layer actuators containing CNT-s, EMIBF4, PVDF-HFP
Preparation of actuator electrode layers:
- CNT(ID-PO356) 50.04 mg
- EMIBF4 (Aldrich) 124.5 mg
- PVDF-HFP (Kynar 2801) 50.1 mg
- DMAc ~(4 mL + 5 mL)
The electrode layer was fabricated by casting 1.6 ml of the electrolyte solution in the Teflon mold (an area of 2.5cm×2.5cm) and evaporating the solvent, perfectly. The thickness of the obtained electrode film was 50–60 μm.
Preparation of actuator electrode layers:
- EMIBF4 (Aldrich) 202 mg
- PVDF-HFP (Kynar 2801) 204,3 mg
- PC 497 mg
- MP 2 mL
The gel electrolyte layers were fabricated by casting 0.3 ml of the solution to the Tefon mold (an area of 2.5cm×2.5 cm) and evaporating the solvent perfectly. The thickness of the obtained gel electrolyte film was 15-18 μm. An actuator film was fabricated by hot-pressing the electrode and electrolyte layers which have the same internal IL. The thickness of the actuator film was 100-110 μm, which are smaller than the sum of those of two electrode and one electrolyte layers, since the thickness of each layer decreases by being hotpressed
Actuator performance analysis
Ionic conductivity of electrolyte layer was calculated from data obtained from electrochemical impedance spectroscopy (EIS) measurements. R value was determined from Nyquist`s plot.
[math]κ=\frac{d(cm)}{S(cm^2)×R(Ω)}\,[/math]