Mediawiki/index.php/EAPedia: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
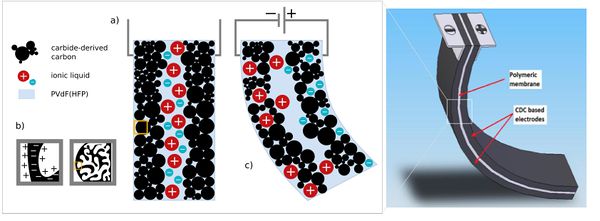
Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications. '''Carbide-derived carbon''' (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors (e.g. SiC, TiC). When the porous carbon-based electrode is immersed into an electrolyte solution (''i'') or electrolyte is introduced into the porous carbon material (''ii''), charge is accumulated at the interface due to the presence of mobile electronic and ionic charge carriers. As seen in Fig. 1 the design of ionic polymer actuators is comparable to electrical double-layer capacitors, often referred as supercapacitors or ultracapacitors. Supercapacitors are highly capacitive devices famous for their high power densities and remarkable energy conversion efficiency up to 98% during the charging-discharging cycle. | Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications. '''Carbide-derived carbon''' (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors (e.g. SiC, TiC). When the porous carbon-based electrode is immersed into an electrolyte solution (''i'') or electrolyte is introduced into the porous carbon material (''ii''), charge is accumulated at the interface due to the presence of mobile electronic and ionic charge carriers. As seen in Fig. 1 the design of ionic polymer actuators is comparable to electrical double-layer capacitors, often referred as supercapacitors or ultracapacitors. Supercapacitors are highly capacitive devices famous for their high power densities and remarkable energy conversion efficiency up to 98% during the charging-discharging cycle. | ||
[[File:EAPEDIA.jpg|600px|thumb|right|Figure 1. ]] | [[File:EAPEDIA.jpg|600px|thumb|right|Figure 1. ]] | ||
===Electrochemical origin of porous carbon-based actuators=== | |||
Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively polarised electrode (anions accumulation). This makes possible to construct three-layered bending device, similar to CNT actoators and other carbon-based actuators. | Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively polarised electrode (anions accumulation). This makes possible to construct three-layered bending device, similar to CNT actoators and other carbon-based actuators. | ||
Revision as of 18:58, 1 April 2013
Carbide-derived Carbon-based Actuators
Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications. Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors (e.g. SiC, TiC). When the porous carbon-based electrode is immersed into an electrolyte solution (i) or electrolyte is introduced into the porous carbon material (ii), charge is accumulated at the interface due to the presence of mobile electronic and ionic charge carriers. As seen in Fig. 1 the design of ionic polymer actuators is comparable to electrical double-layer capacitors, often referred as supercapacitors or ultracapacitors. Supercapacitors are highly capacitive devices famous for their high power densities and remarkable energy conversion efficiency up to 98% during the charging-discharging cycle.
Electrochemical origin of porous carbon-based actuators
Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively polarised electrode (anions accumulation). This makes possible to construct three-layered bending device, similar to CNT actoators and other carbon-based actuators.