Material study of conducting polymers: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
===Conducting polymers=== | ===Conducting polymers=== | ||
It was long thought that being insulators is an integral property of all polymeric materials. The field of conducting polymers or so-called “synthetic metals” was brought into being by the discovery of the electrically conductive properties of polyacetylene in 1977, soon many others followed. The electrochemical synthesis of conductive polypyrrole was reported by Diaz et. al. in 1979. Since then the field has grown immensely, culminating in the year of 2000, when the Nobel Prize of chemistry was awarded to the founders of the field - Heeger, MacDiarmid, and Shirakawa. | It was long thought that being insulators is an integral property of all polymeric materials. The field of conducting polymers or so-called “synthetic metals” was brought into being by the discovery of the electrically conductive properties of polyacetylene in 1977, soon many others followed. The electrochemical synthesis of conductive polypyrrole was reported by Diaz et. al. in 1979. Since then the field has grown immensely, culminating in the year of 2000, when the Nobel Prize of chemistry was awarded to the founders of the field - Heeger, MacDiarmid, and Shirakawa. | ||
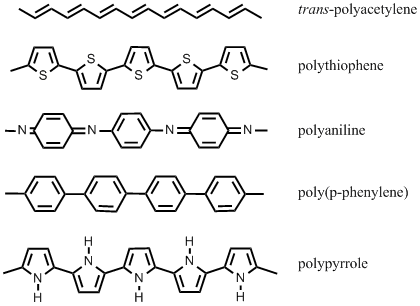
A backbone of conjugated pi-electrons on alternating single and double bonds appears as a common feature of the structures of all these conducting polymers. | A backbone of conjugated pi-electrons on alternating single and double bonds appears as a common feature of the structures of all these conducting polymers. | ||
[[Image:CP-struct.png|center]] | |||
Although being called ‘conducting”, at room temperature and in their native (undoped) state, most of these polymers are insulators. However, the conductivity can often be increased significantly by means of chemical or electrochemical oxidation (p-doping) or reduction (n-doping). The mechanism of conduction in such polymers is quite complex and a generally accepted description is still to be established. | Although being called ‘conducting”, at room temperature and in their native (undoped) state, most of these polymers are insulators. However, the conductivity can often be increased significantly by means of chemical or electrochemical oxidation (p-doping) or reduction (n-doping). The mechanism of conduction in such polymers is quite complex and a generally accepted description is still to be established. | ||
===Polypyrrole=== | ===Polypyrrole=== | ||
| Line 18: | Line 24: | ||
• The stability and resistance of the material to over-oxidation | • The stability and resistance of the material to over-oxidation | ||
The goal is to be able to put together recipes for obtaining polymers with pre-given properties for various applications. | The goal is to be able to put together recipes for obtaining polymers with pre-given properties for various applications. | ||
===People=== | |||
* [[User:Tarmo|Tarmo Tamm]] | |||
* [[User:Urmas|Urmas Johanson]] | |||
* [[User:Rauno|Rauno Temmer]] | |||
Latest revision as of 09:55, 16 March 2012
Conducting polymers
It was long thought that being insulators is an integral property of all polymeric materials. The field of conducting polymers or so-called “synthetic metals” was brought into being by the discovery of the electrically conductive properties of polyacetylene in 1977, soon many others followed. The electrochemical synthesis of conductive polypyrrole was reported by Diaz et. al. in 1979. Since then the field has grown immensely, culminating in the year of 2000, when the Nobel Prize of chemistry was awarded to the founders of the field - Heeger, MacDiarmid, and Shirakawa. A backbone of conjugated pi-electrons on alternating single and double bonds appears as a common feature of the structures of all these conducting polymers.
Although being called ‘conducting”, at room temperature and in their native (undoped) state, most of these polymers are insulators. However, the conductivity can often be increased significantly by means of chemical or electrochemical oxidation (p-doping) or reduction (n-doping). The mechanism of conduction in such polymers is quite complex and a generally accepted description is still to be established.
Polypyrrole
Polypyrrole is, undoubtedly, one of the most studied heterocyclic conducting polymers. The polymer is readily obtained by means of chemical or electrochemical oxidation of the monomer pyrrole. The properties of the material may be tuned at a wide range by adjusting the conditions of the synthesis. This makes the comparison of the results of different research groups rather difficult. Polypyrrole is also available commercially, and finds use in a variety of applications (electronics, displays, coatings, sensors, etc.). Some more recently proposed applications rely on the fascinating bio-compatibility and electro-mechanic properties of polypyrrole. Despite all the research that has been made, there is still a lot to be learned about the formation of the properties of polypyrrole before it can successfully be used in all the areas.
Goals
Electrochemical, spectroscopic, and theoretical investigations performed in our group contribute to the overall understanding of this interesting material. Our interests in particular: • The influence of the dopant ions to the properties. • The mobility of ions inside polymer film and factors influencing that. • Re-doping processes • The stability and resistance of the material to over-oxidation The goal is to be able to put together recipes for obtaining polymers with pre-given properties for various applications.