Mediawiki/index.php/EAPedia: Difference between revisions
No edit summary |
No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Carbide-derived Carbon-based Actuators== | ==Carbide-derived Carbon-based Actuators== | ||
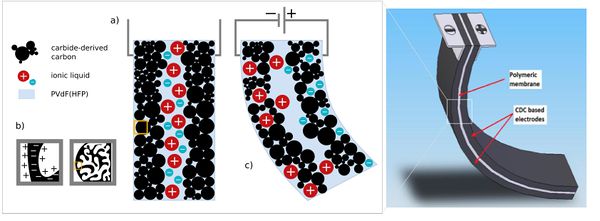
Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications. '''Carbide-derived carbon''' (CDC) | Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications<sup>[[#Ref1|1]]</sup><sup>, </sup><sup>[[#Ref2|2]]</sup><sup>, </sup><sup>[[#Ref3|3]]</sup><sup>, </sup><sup>[[#Ref4|4]]</sup>. '''Carbide-derived carbon''' (CDC) known also as tunable nanoporous carbon is the common term for carbon materials derived from carbide precursors (e.g. SiC, TiC)<sup>[[#Ref1|1]]</sup>, <sup>[[#Ref5|5]]</sup>. CDC materials attracted tremendous attention in the last few years because of their high specific surface areas (''SSA'', 300–2300 m<sup>2</sup>/g) with a broad range of pore sizes (0.3–30 nm)<sup>[[#Ref6|6]]</sup>. Due to the highly tunable porosity in form of high surface areas with very small pores, the CDC materials seem to be very promising for actuator applications<sup>[[#Ref4|4]]</sup>. When the porous carbon-based electrode is immersed into an electrolyte solution (''i'') or electrolyte is introduced into the porous carbon material (''ii''), charge is accumulated at the interface due to the presence of mobile electronic and ionic charge carriers<sup>[[#Ref2|2]]</sup> as seen in Fig. 1 the design of ionic polymer actuators is comparable to electrical double-layer capacitors, often referred as supercapacitors or ultracapacitors. Supercapacitors are highly capacitive devices famous for their high power densities and remarkable energy conversion efficiency up to 98% during the charging-discharging cycle. | ||
[[File:EAPEDIA.jpg|600px|thumb|right|Figure 1. ]] | [[File:EAPEDIA.jpg|600px|thumb|right|Figure 1. Architecture of CDC based actuators. Schematic of charge distribution of device while external voltage is not applied. Notion of electrical double-layer formation during charging inside porous media of CDC (b). Strain of actuator under applied voltage (c). ]] | ||
====Electrochemical origin of porous carbon-based actuators==== | ====Electrochemical origin of porous carbon-based actuators==== | ||
Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively | Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively polarized electrode (anions accumulation)<sup>[[#Ref2|2]]</sup>, <sup>[[#Ref7|7]]</sup>, <sup>[[#Ref8|8]]</sup>. This makes possible to construct three-layered bending device, similar to CNT actuators and other carbon-based actuators<sup>[[#Ref8|8]]</sup>. | ||
By its nature, the three-layered CDC-based bending device has two features when voltage is applied. Firstly, it is a bending-type electrochemical actuator with reversible actuation properties in volage | By its nature, the three-layered CDC-based bending device has two features when voltage is applied. Firstly, it is a bending-type electrochemical actuator with reversible actuation properties in volage range between -2.5 V and +2.5 V. Besides the external shape-changig properties, this device is also an electrochemical capacitor, providing opportunity to store a considerable amount of charge. The design of CDC-based flexible, three-layer actuator is comparable to electrochemical capacitors. The working mechanism is similar, as well as driving voltage (below 3.5 V). However, components of supercapacitors are mounted into hermetically sealed cell to provide high power-densities, long lifetimes, and remarkable energy conversion efficiency. | ||
====Actuator preparation==== | |||
The bending actuator device for open-air applications was obtained by hot-pressing CDC-based electrodes (PVdF was used as binder) and IL containing polymer membrane (PVdF) was sandwiched between two electrode layers. One of the advantages of the CDC-based three-layered composite as an actuator material is that it can be easily formed into various shapes, its properties can be engineered and it can potentially be integrated with MEMS, sensors and control devices to produce smart systems. Forming the self-standing separator layer and electrode layers can be easily made by casting the dispersing solution and evaporating the solvent completely. This simple route offers sufficient control over the thickness of resulting CDC layer. CDC synthesized from titanium carbide (TiC) is widely used electrode material for actuator applications. Following is the example for preparation of reliable actuator based on TiC-800 CDC (TiC is chlorinated at 800 °C).The electrode layer was composed of 20 wt% of CDC, 48 wt% of EMIBF<sub>4</sub>, and 32 wt% of PVdF(HFP). The mixture was stirred at room temperature for 3 days and further was mixture processed in ultrasonic bath for 24 h. The electrode film was made by casting CDC suspension into a Teflon mold and then drying the mixture at 80 ˚C in vacuum oven to remove residues of solvent. The electrolyte layer was prepared from PVdF(HFP) and EMIBF<sub>4</sub> with weight ratio 50/50 in dry film. PVdF(HFP) was dissolved in dimethylacetamide (DMAc) under magnetic stirring at 70 °C. After EMIBF<sub>4</sub> addition, the mixture was stirred for two hours and then poured out into Teflon mold. Finally, the electrolyte film was sandwiched between two electrode films and hot-pressed to connect the layers together. | |||
'''References''' | |||
<span id="Ref1"><sup>1</sup> Y. Gogotsi (ed.), Nanomaterials Handbook (CRC Press, Boca Raton, 2006) p. 239.</span> | |||
<span id="Ref2"><sup>2</sup> B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer Academic, New York, 1999)</span> | |||
<span id="Ref3"><sup>3</sup> M. Hahn, O. Barbieri, R. Gallay, and R. Kötz, Carbon 44, 2523 (2006).</span> | |||
<span id="Ref4"><sup>4</sup> J. Torop, V. Palmre, M. Arulepp, T. Sugino, K. Asaka, A. Aabloo, Carbon 49, 3113 (2011).</span> | |||
<span id="Ref5"><sup>5</sup> M. Arulepp, J. Leis, M. Lätt, F. Miller, K. Rumma, E. Lust, and A. F. Burke, J. Power Sources 162, 1460 (2006)</span> | |||
<span id="Ref6"><sup>6</sup>Thomberg, T.; Kurig, H.; Jänes, A.; Lust, E. (2011). Mesoporous carbide-derived carbons prepared from different chromium carbides. Microporous and Mesoporous Materials, 141(1-3), 88 - 93.</span> | |||
<span id="Ref7"><sup>7</sup> J. Huang, B. G. Sumpter, and V. A. Meunier, Chem. Eur. J. 14, 6614 (2008).</span> | |||
<span id="Ref8"><sup>8</sup>T. Fukushima, K. Asaka, A. Kosaka, and T. Aida, Angew. Chem. Int. Ed. 44, 2410 )2005).</span> | |||
<span id="Ref1"><sup>1</sup>X</span> | |||
<span id="Ref1"><sup>1</sup>X</span> | |||
Latest revision as of 08:54, 20 May 2013
Carbide-derived Carbon-based Actuators
Non-faradaic charge storage mechanism, where chemisorbed ions reside on porous electrode to form an electrochemical double-layer (EDL) holds a great potential not only in EDL capacitor applications, but also actuator applications1, 2, 3, 4. Carbide-derived carbon (CDC) known also as tunable nanoporous carbon is the common term for carbon materials derived from carbide precursors (e.g. SiC, TiC)1, 5. CDC materials attracted tremendous attention in the last few years because of their high specific surface areas (SSA, 300–2300 m2/g) with a broad range of pore sizes (0.3–30 nm)6. Due to the highly tunable porosity in form of high surface areas with very small pores, the CDC materials seem to be very promising for actuator applications4. When the porous carbon-based electrode is immersed into an electrolyte solution (i) or electrolyte is introduced into the porous carbon material (ii), charge is accumulated at the interface due to the presence of mobile electronic and ionic charge carriers2 as seen in Fig. 1 the design of ionic polymer actuators is comparable to electrical double-layer capacitors, often referred as supercapacitors or ultracapacitors. Supercapacitors are highly capacitive devices famous for their high power densities and remarkable energy conversion efficiency up to 98% during the charging-discharging cycle.
Electrochemical origin of porous carbon-based actuators
Charge induced electromechanical actuation is a combination of charge injection during electrical double-layer charging of high surface area carbon, and ion migration/accumulation induced by charge distribution. Both effects result in the more expressed expansion of the negatively polarized electrode (cations accumulation) compared to the positively polarized electrode (anions accumulation)2, 7, 8. This makes possible to construct three-layered bending device, similar to CNT actuators and other carbon-based actuators8. By its nature, the three-layered CDC-based bending device has two features when voltage is applied. Firstly, it is a bending-type electrochemical actuator with reversible actuation properties in volage range between -2.5 V and +2.5 V. Besides the external shape-changig properties, this device is also an electrochemical capacitor, providing opportunity to store a considerable amount of charge. The design of CDC-based flexible, three-layer actuator is comparable to electrochemical capacitors. The working mechanism is similar, as well as driving voltage (below 3.5 V). However, components of supercapacitors are mounted into hermetically sealed cell to provide high power-densities, long lifetimes, and remarkable energy conversion efficiency.
Actuator preparation
The bending actuator device for open-air applications was obtained by hot-pressing CDC-based electrodes (PVdF was used as binder) and IL containing polymer membrane (PVdF) was sandwiched between two electrode layers. One of the advantages of the CDC-based three-layered composite as an actuator material is that it can be easily formed into various shapes, its properties can be engineered and it can potentially be integrated with MEMS, sensors and control devices to produce smart systems. Forming the self-standing separator layer and electrode layers can be easily made by casting the dispersing solution and evaporating the solvent completely. This simple route offers sufficient control over the thickness of resulting CDC layer. CDC synthesized from titanium carbide (TiC) is widely used electrode material for actuator applications. Following is the example for preparation of reliable actuator based on TiC-800 CDC (TiC is chlorinated at 800 °C).The electrode layer was composed of 20 wt% of CDC, 48 wt% of EMIBF4, and 32 wt% of PVdF(HFP). The mixture was stirred at room temperature for 3 days and further was mixture processed in ultrasonic bath for 24 h. The electrode film was made by casting CDC suspension into a Teflon mold and then drying the mixture at 80 ˚C in vacuum oven to remove residues of solvent. The electrolyte layer was prepared from PVdF(HFP) and EMIBF4 with weight ratio 50/50 in dry film. PVdF(HFP) was dissolved in dimethylacetamide (DMAc) under magnetic stirring at 70 °C. After EMIBF4 addition, the mixture was stirred for two hours and then poured out into Teflon mold. Finally, the electrolyte film was sandwiched between two electrode films and hot-pressed to connect the layers together.
References
1 Y. Gogotsi (ed.), Nanomaterials Handbook (CRC Press, Boca Raton, 2006) p. 239.
2 B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer Academic, New York, 1999)
3 M. Hahn, O. Barbieri, R. Gallay, and R. Kötz, Carbon 44, 2523 (2006).
4 J. Torop, V. Palmre, M. Arulepp, T. Sugino, K. Asaka, A. Aabloo, Carbon 49, 3113 (2011).
5 M. Arulepp, J. Leis, M. Lätt, F. Miller, K. Rumma, E. Lust, and A. F. Burke, J. Power Sources 162, 1460 (2006)
6Thomberg, T.; Kurig, H.; Jänes, A.; Lust, E. (2011). Mesoporous carbide-derived carbons prepared from different chromium carbides. Microporous and Mesoporous Materials, 141(1-3), 88 - 93.
7 J. Huang, B. G. Sumpter, and V. A. Meunier, Chem. Eur. J. 14, 6614 (2008).
8T. Fukushima, K. Asaka, A. Kosaka, and T. Aida, Angew. Chem. Int. Ed. 44, 2410 )2005).
1X 1X